Copper metal can be treated so it has surface colors that range from black to green. The color is called a “patina.” The formation of patina on copper is similar to the formation of rust on iron. Both happen when oxygen in the air interacts with metal atoms in the presence of water. Colors on patinated copper jewelry range from blue to green to red to black.
Silver can also be patinated, but the color range is smaller than the range available with copper. Some patination chemicals make brass become fragile and crumbly, so I don’t try to patinate brass.
Sometimes patination methods use chemicals, and sometimes you can create a patina using heat. Regardless of the patination method, this is the patination process:
- Clean the piece to be patinated. I use rubbing alcohol.
- Patinate the piece
- If desired, polish textured highlights
- Apply a protective coating. You need a coating to stop the patina from coming off on your skin. I use either Renaissance Wax or ProtectaClear (www.everbritecoatings.com). You can bake on the hypo-allergenic ProtectaClear and it seems to last longer than wax. ProtectaClear is available as a liquid (dip your pieces in it to coat them) or a spray.



Chemical Patination
There are at least five different ways to apply patination chemicals to copper:
Cold Fuming. Place the jewelry on a platform (like a plastic lid) to keep it out of the patination liquid in a closed plastic container or bag. Pour the patination solution on the bottom of the container. Seal the container and let the solution oxidize the copper. Cold fuming works well for ammonia and vinegar.
Painted Solutions. Paint the patination solution onto the jewelry with a swab or brush. Allow the metal to dry, and repeat the process until you like the color. This method is used with salt and cupric nitrate solutions,
Sprayed Solutions. This method may be used to create an even or speckled effect. Multiple applications may be needed. Keep applications light. Spraying works well for salt and cupric nitrate solutions.
Bound Materials. You can bind feathers and plants with string or thread. The bound items act as resists. The jewelry is immersed in the patination solution. This method may work with salt solutions, but not ammonia or vinegar.
Moistened Shavings. Damp (not wet) saw dust, tobacco, hamster litter or other absorbant materials work well for creating speckled patination patterns. Possible absorbant materials include resin-free sawdust and shavings (no oak either), peanut shells, kitty litter, styrofoam balls or chunks, crushed cork, sisal or coarse fibers, pine needles, grass, leaves, straw, etc. Use in a sealed container. The pieces may be shaken to obtain a randomized effect.
You can buy commercial products to create patinas on copper. Some items advertised as patina agents are actually stains (for example, Swellegant and Ranger Vintag products). JAX makes green and black patina solutions for copper. Novacan makes a blackener.
Traditionally, liver of sulfur is used to blacken copper. Liver of sulfur is a mix of chemicals that contain sulfur. It comes in dry, liquid, and gel. Gel is my favorite. The mix may include potassium sulfide, potassium polysulfide, potassium thiosulfate, and potassium bisulfide. Liver of sulfur fumes are not good to breathe, so you might consider working outdoors with it. You need to neutralize the used liver of sulfur solution with baking soda. Add baking soda to the solution until it no longer foams, then flush it down the toilet. Lily-Tree (https://www.youtube.com/watch?v=3NjRUpi8xmc)has a nice video on using liver of sulfur.
You can make your own patination solutions. Household items like ammonia, vinegar, and salt can be used to color copper. TheCrafsMan (https://www.youtube.com/watch?v=yl67z6XmNiQ) has a fun and practical YouTube video on how to patinate with household items, as well as with liver of sulfur.
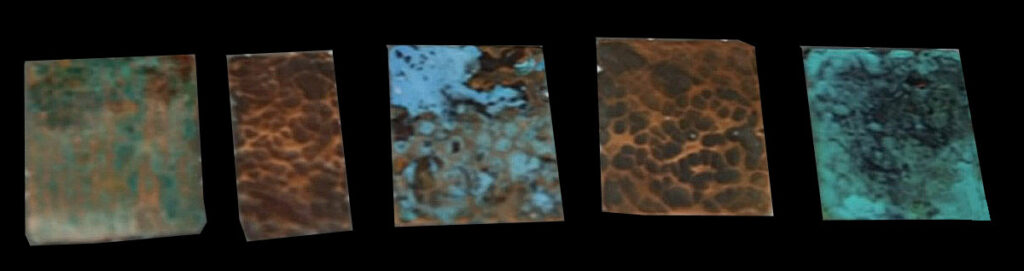
My favorite green patination method for copper uses ammonium chloride (a fertilizer, you can buy it online) and sawdust or tobacco. Fill a small plastic container, with a lid, halfway with sawdust. Dissolve 1.5 tablespoons of ammonium chloride in 0.5 cup of water. Pour the ammonium chloride solution into the sawdust.

Seal the container and gently shake to mix the liquid with the sawdust. Once the sawdust is thoroughly damp (make some more ammonium chloride solution and add it to the sawdust, if needed), place your copper jewelry under the sawdust, and seal the container. 
Allow the copper to sit in the container for about an hour, then check the color. Let the copper patina develop until you get the color you want. Don’t let it sit too long or the finish will be flakey.

Copper Patination with Heat
Sometimes I want to create a red color on copper without the bother of using a chemical. The results with heat are less predictable than using chemicals, but that means each piece you create is definitely one of a kind! There are several online videos about heat coloring copper, but this one from the Online Jewelry Academy focuses on jewelry (not sculpture) and managing costs: https://www.youtube.com/watch?v=2X16hNlc_5I
Basically, you heat the copper piece to orange hot with a torch, then dunk the piece in boiling water. That’s it! A dark red patina develops . I used heat patina to color the copper background of this brass and copper ichthyosaur pin. The patina was sealed with ProtectaClear.

Protecting Your Patinated Jewelry
Avoid showering, bathing, or swimming with patinated jewelry. This prevents absorption of moisture and oils which can change the color. Finally, avoid contact with things that may scratch the piece, including other pieces of jewelry.
